

Monitoring protein conformation along the pathway of chaperonin-assisted folding. GroEL stimulates protein folding through forced unfolding. Chaperonin-catalyzed rescue of kinetically trapped states in protein folding. Structural features of the GroEL−GroES nano-cage required for rapid folding of encapsulated protein. Dual function of protein confinement in chaperonin-assisted protein folding. The crystal structure of the asymmetric GroEL−GroES−(ADP)7 chaperonin complex. Physicochemical determinants of chaperone requirements. A more precise characterization of chaperonin substrates. A systematic survey of in vivo obligate chaperonin-dependent substrates. Chaperonin-mediated protein folding: using a central cavity to kinetically assist polypeptide chain folding. Folding of newly translated proteins in vivo: the role of molecular chaperones. The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. K., De los Rios, P., Christen, P., Lustig, A. How evolutionary pressure against protein aggregation shaped chaperone specificity. A key paper in understanding how chaperones recognize their substrates. Interaction of Hsp70 chaperones with substrates. Chaperone-assisted selective autophagy is essential for muscle maintenance.
DEFINE CHAPERONE PROTEIN DRIVERS
The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Coupled chaperone action in folding and assembly of hexadecameric Rubisco. Molecular chaperones: assisting assembly in addition to folding. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Converging concepts of protein folding in vitro and in vivo. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. 5, 735–740 (2010) This paper provides evidence that the exposure of hydrophobic surfaces by oligomeric aggregation intermediates correlates with their toxicity. ANS binding reveals common features of cytotoxic amyloid species. Protein misfolding, functional amyloid, and human disease. This exciting paper describes, at atomic resolution, the structural features of a non-native folding intermediate that are critical for amyloidogenic aggregation.Ĭhiti, F. Conformational conversion during amyloid formation at atomic resolution. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cellular mechanisms of membrane protein folding. This seminal study puts forward the idea that chaperones function in buffering the otherwise deleterious consequences of mutations. Hsp90 as a capacitor for morphological evolution. Chaperonin overexpression promotes genetic variation and enzyme evolution. Protein aggregation in crowded environments. Equilibrium intermediates in the reversible unfolding of firefly ( Photinus pyralis) luciferase. Molecular chaperones in cellular protein folding.
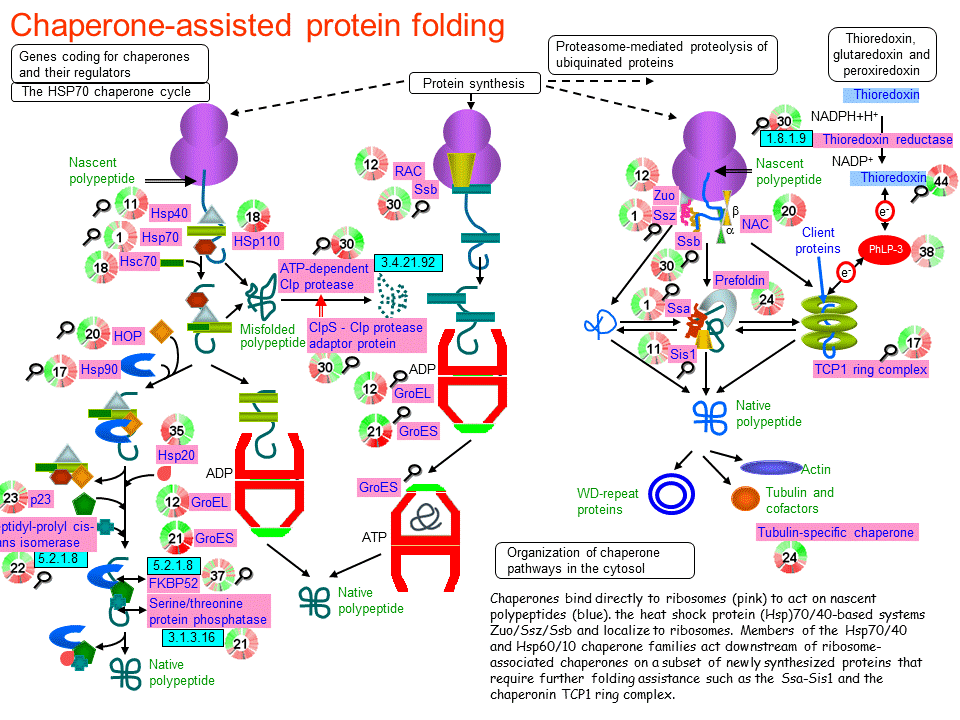
Adapting proteostasis for disease intervention. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Biological and chemical approaches to diseases of proteostasis deficiency. Function and structure of inherently disordered proteins. An expanding arsenal of experimental methods yields an explosion of insights into protein folding mechanisms. Protein folding - a perspective from theory and experiment.


 0 kommentar(er)
0 kommentar(er)
